The following case study demonstrates how the Caleva Mixer Torque Rheometer. The MTR-3 was an invaluable aid in a specific case. A manufacturer of generic Venlafaxine pellets was experiencing inconsistent results from their manufacturing line. The resultant product was variable in both usable yield (weight of acceptable pellets as a % of weight of granulation made), and dissolution profile. The product was a registered pharmaceutical product with a 15% water content. It was not known why, or how, this liquid binder content was determined to be the optimum.
Since the formulation was already registered, neither the formulation ingredients nor the amount of liquid binder could be easily modified. The texture of the extrudate was visually inconsistent but no quantitative assessment of this casual observation had been undertaken. To assess this issue, the rheological properties of the customer’s granulation were examined in a quantitative way. From a practical standpoint this was only possible using the Caleva Mixer Torque Rheometer (MTR-3).
TRIALS
Two key parameters were examined with the MTR-3.
OPTIMUM BINDER CONTENT
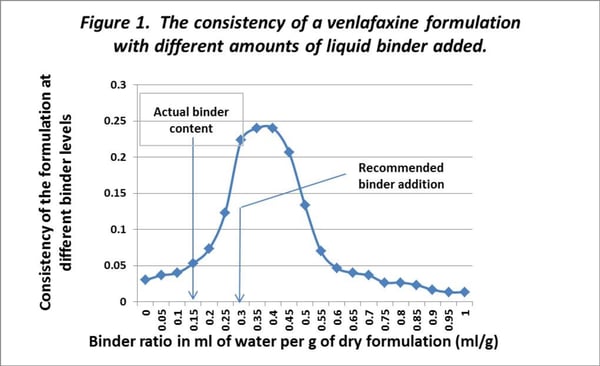
An initial trial was performed to look at the effect of binder content on the consistency of the granulation. This was clearly recognised as important but in reality, could not be easily changed. In the original development the optimum binder concentration was determined by the “hand squeeze test” (a subjective assessment and not really appropriate for the 21st century pharmaceutical industry!). The effect of different amounts of binder content on the consistency of a granulation was assessed using a 20g (dry powder weight) sample. The results are shown in figure 1.
GRANULATION TIME
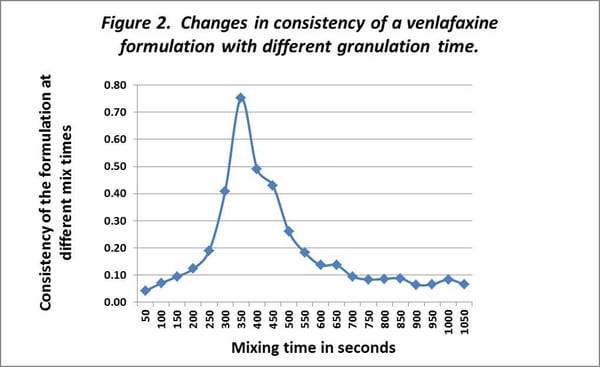
In the original development, no quantitative assessment had been undertaken of the influence of mixing upon the formulation properties, possibly because it was assumed that no appropriate tool was available to provide a quantitative measurement or that this factor was considered to be of little significance to the final formulation providing it was granulated “enough”. Within the registration, some flexibility was permitted in the amount and intensity of mixing. There was scope for changes to be made in this area. An assessment was made using an MTR-3 of the effect of mixing on the consistency of the formulation. This assessment was again made utilizing a 20g (powder dry weight) sample. The instrument was used to measure the consistency of the material in the bowl as the granulation progressed. The results are shown in Figure 2.
RESULTS
Binder Content
For pellet formulations, an ideal % binder content is generally somewhere just before the maximum consistency reading is reached. These results show that the 15% binder content that is used in this formulation is unlikely to be optimum for this product. In this case the results suggest that a water content of about 35% is optimum in this formulation. However, as the formulation was already registered with the authorities with a water content of 15% the manufacturer, understandably did not want to change this value. The early assessment made using the “hand squeeze test” and without any quantitative data is inadequate and is likely to be a contributing factor to the quality problems encountered in production. The formulation will never be “optimum” as the binder ratio cannot practically be changed due to product registration.
A similar problem was apparent with the granulation time given to the formulation. The results (Figure 2 above) show that the granulation regime was of paramount importance if consistent production quality was to be realized in practise.
DISCUSSION
Quantifiable measurements of formulation consistency are a necessary (or at the very least desirable) part of formulation development if issues with formulations are to be minimized and optimum yields achieved once they get into production. Whilst the importance of the percentage of binder liquid added to a formulation is recognised as important to achieve optimum production quality, many formulators who work in formulation development still rely on subjective and non-quantifiable assessments. The importance of the granulation program is sometimes not recognised as a significant factor in formulation development. Contrary to this general perception the granulation process should be consistent in its operation and assessed in a quantitative way as a standard part of product development. When used as a fundamental part of formulation development processes the Caleva Mixer Torque Rheometer can be instrumental in avoiding later problems in production and is the collection of quantitative data recommended as a standard part of the development process.
CONCLUSIONS
There are three main ways available to determine the optimum ratio and mix time:-
- Adding binder and testing by ‘eye and hand’ to reach an estimated optimum ratio. Obvious limitations and no reproducibility!
- The systematic investigation of a range of possible options with subsequent testing of the final characteristics of tablets and spheroids (pellets) produced. A time-consuming and laborious undertaking!
- Using the Caleva Mixer Torque Rheometer (MTR-3) the only method that is objective, repeatable, quantifiable and rapid.



Leave A Comment